WASHINGTON — A Maryland-based company says the COVID-19 vaccine it is developing is showing promise against strains of the deadly pandemic disease.
Over the last few months, variants of the coronavirus have appeared in countries like the United Kingdom and South Africa. Experts say some coronavirus variants have proven to be more transmissible than the original virus.
Novavax, a biotechnology company based in Gaithersburg, recently reported findings from its vaccine the United Kingdom Phase 3 trial.
In a statement, the company said its vaccine demonstrated an 89.3% efficacy rate in the United Kingdom trial.
Furthermore, Novavax said its vaccine showed an efficacy rate of 95.6% against the original COVID-19 strain and an efficacy rate of 85.6% against the UK variant strain.
Novavax also revealed the results of its South Africa Phase 2B trial. It said the vaccine showed an efficacy rate of around 60% in the pool of trial participants.
Novavax said data from that trial suggested prior infection with COVID-19 may not completely protect against subsequent infection by the South Africa escape variant. However, it added vaccination with its vaccine did provide “significant protection”.
“[Our vaccine] has the potential to play an important role in solving this global public health crisis,” said Novavax CEO and President Stanley Erck. “We look forward to continuing to work with our partners, collaborators, investigators and regulators around the world to make the vaccine available as quickly as possible.”
Novavax Research and Development President Dr. Gregory Glenn participated in a virtual symposium organized by the New York Academy of Sciences Tuesday. According to the academy, Dr. Glenn said he expects the US Food and Drug Administration to approve the company’s vaccine soon.
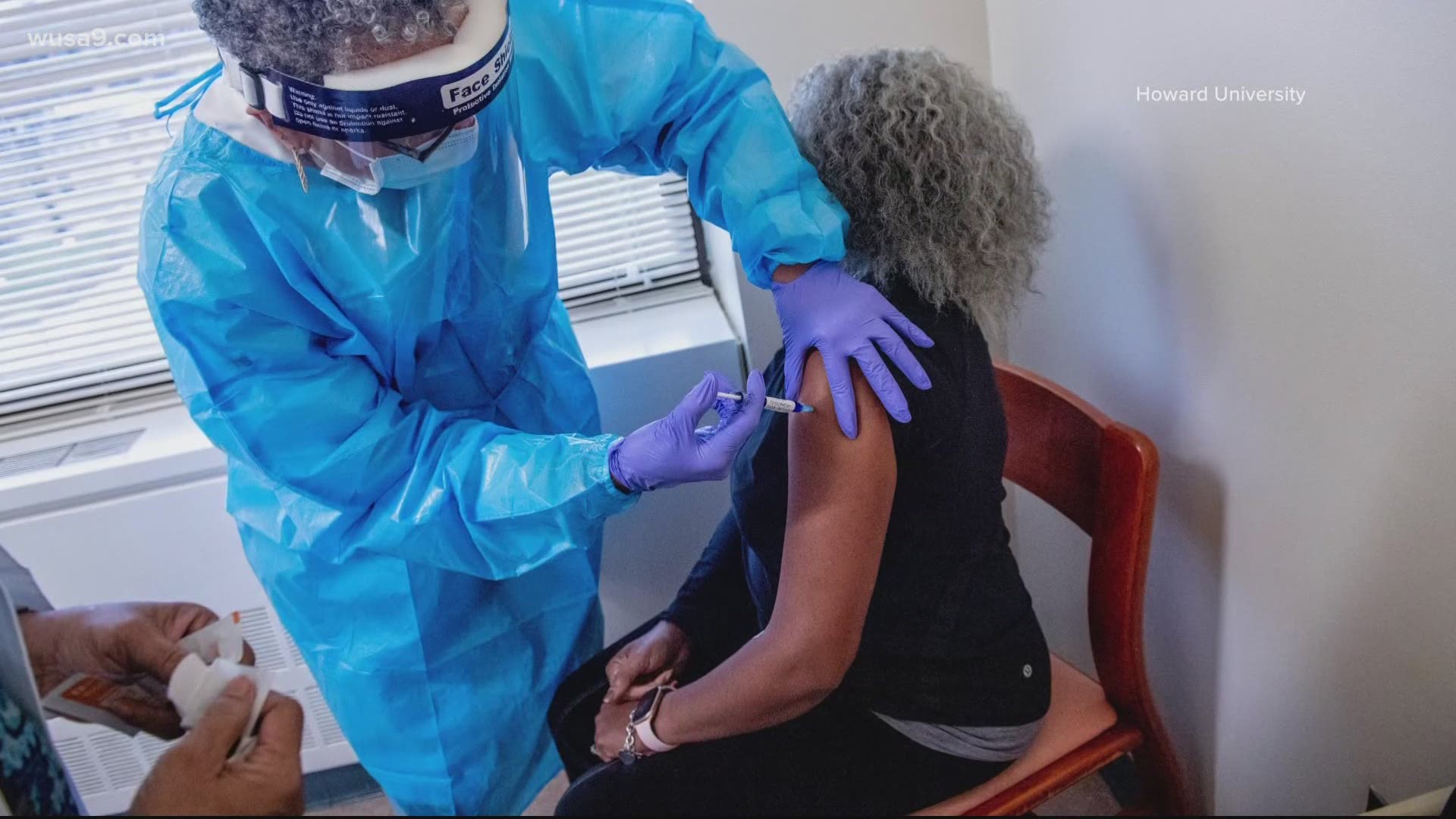
Howard University is participating as a Phase 3 COVID clinical trial site to also evaluate the safety and effectiveness of the Novavax vaccine.
The university said its involvement as a trial site was also strongly motivated by concerns regarding the coronavirus in the Black and Latino communities.
Alexandria resident and Howard University alum Darnella McGuire-Nelson has participated in the trial as a volunteer.
She said she is happy to hear the good news so far regarding the Novavax vaccine especially considering the disproportionate impact the virus has had on America’s minority populations.
“[It is] very encouraging that you are part of a study that will move medicine forward and to help hundreds of thousands, millions of people," she said.
However, Dr. Carla Williams, Associate Professor of Medicine and Public Health at Howard University, adds any vaccine that is produced must be distributed equitably.
“Can't let [equity] be an afterthought or just hope that it's going to happen,” she said. “So, we do need to look at what the patterns have been and make sure that we understand why those exist, and then be very aggressive about addressing those.”
Howard’s Novavax trial is being managed at its clinical research unit at Howard University Hospital and through a mobile unit that drives to different community locations.
If you would like to learn how to become a part of the trial, click here.