The Food and Drug Administration has issued a "do not use" warning for another COVID-19 test, saying it has not been authorized for use in the U.S. and could provide false results. It's at least the third such warning issued for coronavirus tests this year.
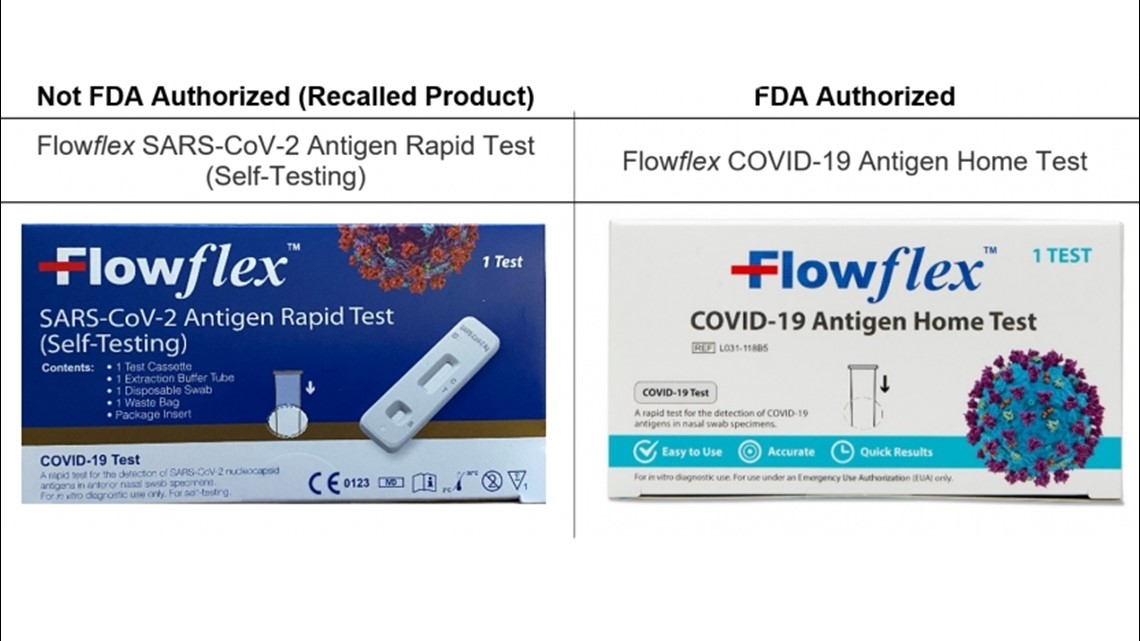
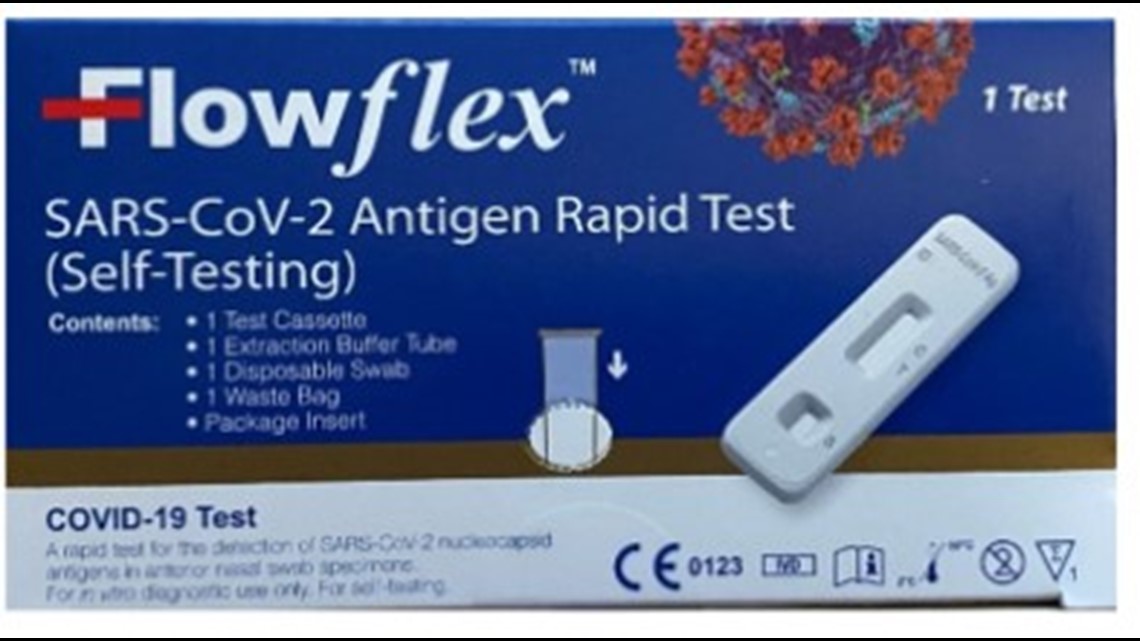
The warning is for the “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” from ACON Laboratories. The test comes in a dark blue box with white lettering and symbols in the lower right corner of the box, including the letters “CE.”
The FDA said it should not be confused with the similarly-named "Flowflex COVID-19 Antigen Home Test," which comes in a white box. That test is authorized by the FDA and can continue to be used.

The concern is the “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” could provide false results. A false positive would mean the test says someone has COVID-19 when they don't. A false negative would indicate the person does not have COVID when they actually do.
ACON recalled the test in the U.S. on Jan. 9. It said the test is only authorized for sale in Europe and other markets.

Health care providers who used the test are urged to consider retesting their patients with a different, authorized test.
The FDA issued a warning on Jan. 11 to stop using the LuSys Laboratories COVID-19 Antigen Test (Nasal/Saliva) and the LuSys Laboratories COVID-19 IgG/IgM Antibody Test over concerns about "high risk of false results."
A similar warning was issued Jan. 28 for Empowered Diagnostics' CovClear COVID-19 Rapid Antigen Test and the ImmunoPass COVID-19 Neutralizing Antibody Rapid Test, also due to concern about false results.

