WASHINGTON — The VERIFY team sorts through misinformation to bring you the facts.
We’ve heard a lot of terms and phrases throughout the COVID-19 pandemic, but not all of us have medical degrees. So what do they all mean? Let’s break it down with some of the top keywords we have heard so far.
SOURCES:
What terms mean what?
IMMUNITY: Protection from a disease. Immunity allows you to be exposed to lower or no risk of catching the disease.
IMMUNOGENICITY: A measurement of the vaccine’s ability to generate an immune response. A quick tip from a VERIFY source: People don’t have immunogenicity, vaccines do.
ENDEMIC: The expected level of disease in a community, or a baseline. These levels always exist, but can change depending on the spread of a disease.
EPIDEMIC: When there is a sudden increase in the number of cases of a certain disease than what's expected for a certain area.
ANTIGENS: Foreign substances that can cause disease in the body, like a virus. The presence of antigens typically triggers the production of antibodies.
ANTIBODY: A protein produced in response to an antigen. Antibodies help destroy the disease. Antibodies are found in the blood.
INCUBATION PERIOD: The time from contact with infectious agents (bacteria or viruses) to onset of disease.
ASYMPTOMATIC: When a patient is a carrier of an illness but doesn't show symptoms.
VACCINE: A product that’s typically injected into the body to stimulate immunity. It can also be administered through the mouth and nose.
PLACEBO: A treatment that’s known to have no effect on humans.
EFFICACY RATE: Measurement of how well a vaccine is at preventing disease.
VACCINE TRIALS: Multi-step processes that test the effectiveness of a vaccine, from clinical trials to placebo monitoring.
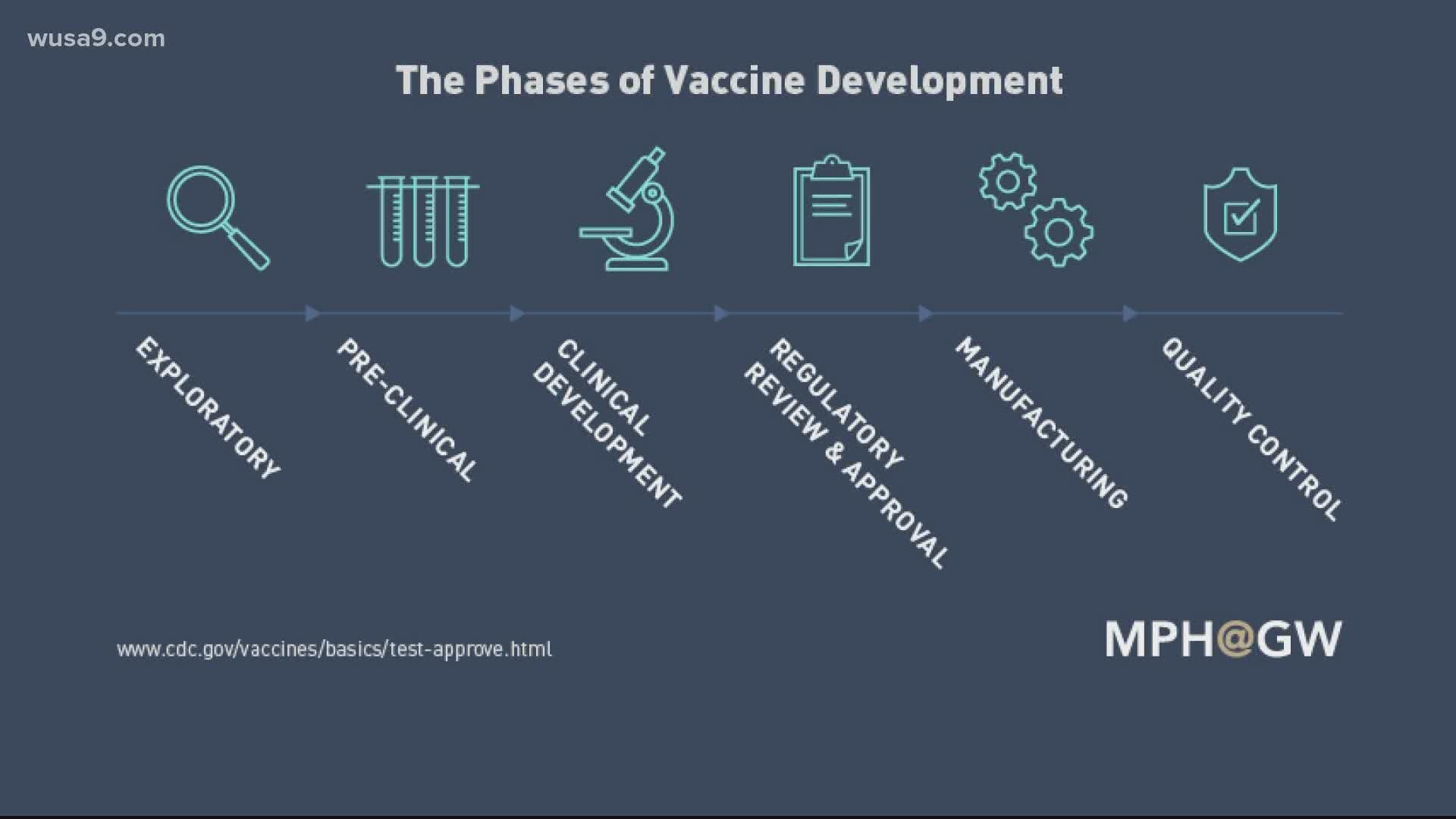
What are do each of the clinical phases for vaccine approval look like?
Phase 1: A small group of volunteers receives the vaccine, around 20-100 people, and scientists learn more about dosage and potential side effects. Scientists may start to learn about the vaccine's effectiveness. If no serious side effects are detected, scientists move on to phase 2.
Phase 2: A larger group of about a couple hundred volunteers participate. Scientists study what the short-term side effects are and see how the dosage relates to an immune response.
Phase 3:Hundreds or thousands of volunteers are observed. Results from vaccinated people are compared to those who received a placebo or some other vaccine, to learn more about side-effects, effectiveness and safety.
"The trials and all other data must show that the vaccine’s benefits outweigh the potential risks for people who will be recommended to receive the vaccine," the FDA writes in the factsheet. "Only if a vaccine’s benefits are found to outweigh its potential risks does the FDA grant a license for the vaccine, allowing it to be used by the public."
After the clinical trials, there are several safety procedures in place to monitor safety and effectiveness, before an EUA is granted.